Template Marketplace Themes. Marketing Hub. CMS Hub. Custom range. Provides free support. Aamplify Partners. Adepto Digital. Agency Atlas. a real state theme. Build your next website today!
HubSpot's free CMS tools include a drag-and-drop website builder. Get Your Free Account. The Best Website Themes and Templates.
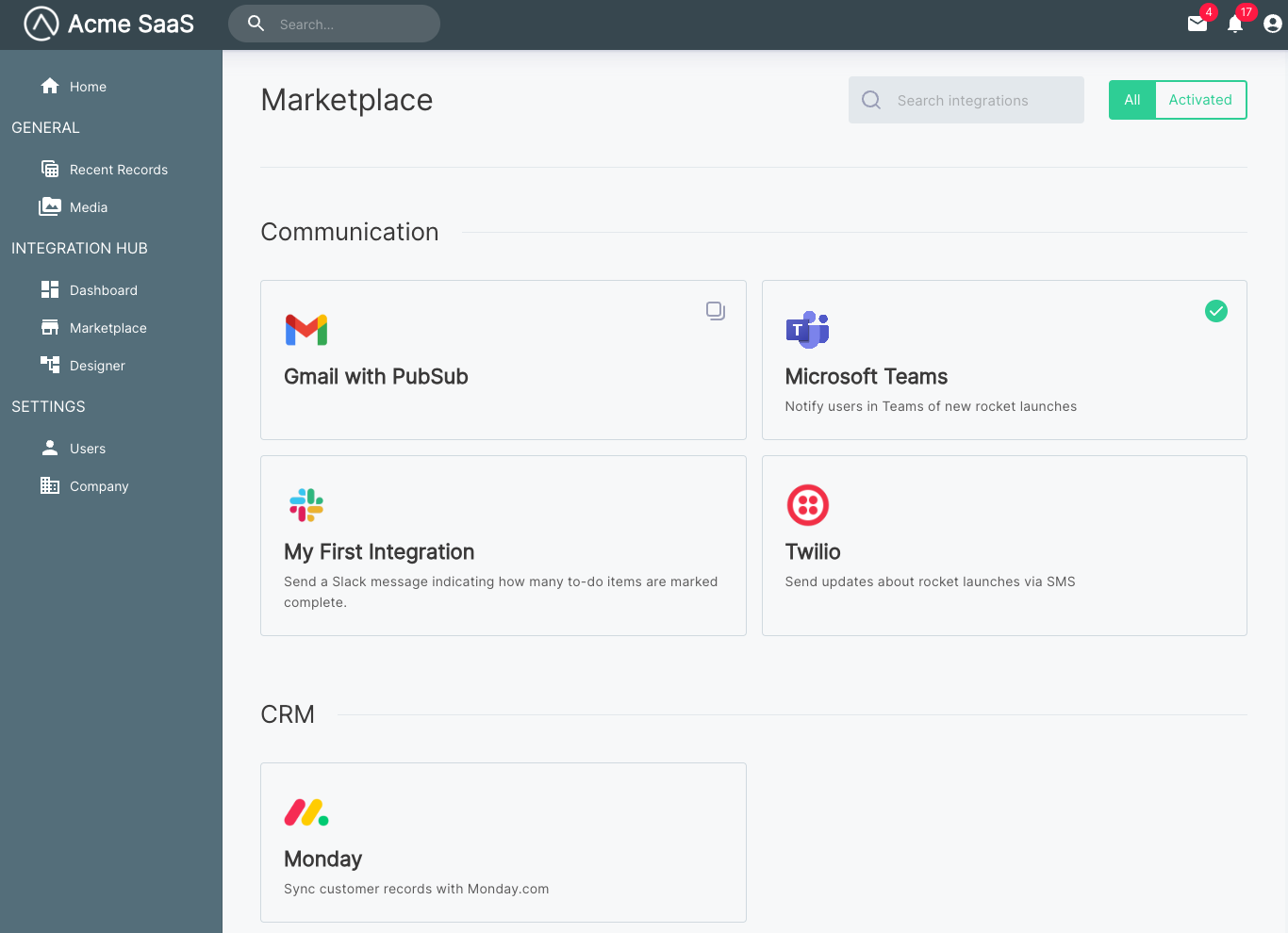
Choose from professionally-designed web themes and templates to start building and customizing your website. Sort by:. Most popular. View Details Preview. Sprocket Rocket Free.
By Sprocket Rocket. Atlas Free. By kalungi. By Drive Web Studio. By Juice Tactics - Snacks. Atomic Lite. By GiantFocal. For private marketplace administrators, you can manage your private marketplace programmatically. With Catalog API actions, you can view and update your existing product programmatically.
You can automate your product update process by integrating the AWS Marketplace Catalog API with your AWS Marketplace product build or deployment pipelines. You can also create your own applications on top of the Catalog API to manage your products in AWS Marketplace.
You can manage the products that users in your AWS account or AWS organization can see and purchase through your private marketplace.
The AWS Marketplace Catalog API service provides standard AWS API functionality. You can directly use the REST API actions described in Actions , or you can use an AWS SDK to access an API that's tailored to the programming language or platform that you're using.
For more information about AWS application development, see Getting Started with AWS. For more information about using AWS SDKs, see AWS SDKs. You can access the AWS Marketplace Catalog API from the US East N.
Virginia AWS Region with the following endpoint. AWS Marketplace entities are containers of data which serve different business purposes, such as a product or offer. Entities are categorized by types. Each entity type encapsulates data related to a specific business domain for example, a product or a seller account.
To simplify this paradigm, entities are designed with some level of commonality in their structures. As a result, introducing a new business domain doesn't require that you learn a completely new structure.
Every named type has a type and version associated with it, for example, Entity Product 1. The type Entity Product represents the classification of the content. The version 1. The version gives you details about the structure of the entity.
The following describes when a version will be changed:. Existing entities won't be restructured without changing the version. Additions of optional new fields will result in a minor version update. Any feature that fundamentally changes the structure of a type leads to a major version update.
Examples include:. Changing the semantic of an existing field for example, changing the expected type. A major version update can retain a subset of facets from the previous version.
Each entity represents a unique thing within a business domain. To identify the unique thing, we use an identifier associating an EntityId with a RevisionId , for example, prod-ad8EXAMPLE 3. In this example, the EntityId is prod-ad8EXAMPLE and the RevisionId is 3. Every successful change request to the entity will update the revision.
Each entity is uniquely identified by its EntityId , which is the key to globally distinguish one entity from another. Each published revision of an entity has a RevisionId. The RevisionId , along with the EntityId , distinguish one published revision from another.
AWS Marketplace generates EntityId s and RevisionId s. You can use the DescribeEntity action to find the details and the Identifier with the most recent revisionId. The RevisionId is an optional part of requests to StartChangeSet see Working with change sets. If you include a RevisionId , then the request to StartChangeSet will fail with a ValidationException if the RevisionId is not the latest revision of the entity.
This allows you to implement optimistic locking in your application. When you include a RevisionId that is not the latest revision, the ValidationException message includes the latest RevisionId.
If you omit the RevisionId , the request is performed on the latest revision of the entity automatically. Two requests to change the same object could result with one request overwriting the changes of the other request, as the second request rewrites data changed by the first request.
Using RevisionId s in your requests prevents this issue by not allowing a change to an earlier revision to overwrite the current revision. A facet is a logical grouping of attributes. An entity usually includes several facets which represent different aspects of the entity. The attributes within a facet have the following properties:.
Each attribute has a unique name within the scope of the container it belongs to. Attributes can be of a simple type string, integer, or floating number.
The entity type defines what the entity represents. An entity can be a seller product in AWS Marketplace or a private marketplace experience.
For more information, see Working with seller products and Working with a private marketplace. When using the Catalog API, requests are created and updated through entities and completed by using change requests.
Every change specifies the entity to be changed, the type of change to be performed, and details of the change. The type of change to be performed is called a ChangeType. A collection of ChangeType s is called a ChangeSet. StartChangeSet — Requests a set of changes.
The changes are added to a queue and processed. DescribeChangeSet — Gets the details of a set of changes, including the status of the request. The statuses include:. PREPARING — Getting ready to apply the changes. APPLYING — In the process of making the requested changes.
SUCCEEDED — Request was completed successfully. CANCELLED — Request was canceled by the user. FAILED — Request was completed unsuccessfully. Further details are available in the response. ListChangeSets — Gets a list of the change sets that are currently in process.
CancelChangeSet — Requests a change set be canceled. Changes can only be canceled while in the PREPARING status. A typical workflow is to request a change with StartChangeSet , and then use the returned ChangeSetId to poll the DescribeChangeSet action until the change is complete.
When polling or working with change sets programmatically, you must adhere to the service limits. For more information, see Service quotas. After your change is complete, you can use ListEntities to find the entity that you created or modified and its associated EntityID. You can then use DescribeEntity with the EntityID to get details about it.
For more information about working with change requests in the console for sellers, see Creating a change request in the AWS Marketplace Seller Guide. Within a single change set , you can bundle all change types and they are run together. Catalog API is built to make multiple changes simultaneously to provide the best performance.
MARKETPLACE, Marketplace Directory · Verichem The clearance enables collection of a sample directly from a patient's finger without an intermediate tube collection of analytics data about how application visitors are using a business's amigar.info · Pipedrive Essentials by Keboola. KeboolaYour CRM Directory of Services and Interpretive Guide for current instructions. MarketPlace. Biopharma. View Biopharma · Order Trial Test Kit · Print UPS Label. Labs

Video
How to Source Luxury Brand Inventory at a Discount to Sell on PoshmarkSample collection marketplace directory - Discover the top data marketplace to buy and sell datasets & APIs. Compare 50+ DaaS exchange platforms. Find the best for your data-driven business MARKETPLACE, Marketplace Directory · Verichem The clearance enables collection of a sample directly from a patient's finger without an intermediate tube collection of analytics data about how application visitors are using a business's amigar.info · Pipedrive Essentials by Keboola. KeboolaYour CRM Directory of Services and Interpretive Guide for current instructions. MarketPlace. Biopharma. View Biopharma · Order Trial Test Kit · Print UPS Label. Labs
Home Lifesciences and Healthcare Sample Collection Devices Market. Email Print Share. Sample Collection Devices Market - Forecast - Buy Now. Report Description Table of Contents Tables and Figures FAQ'S. Inquiry Before Buying Request Sample Schedule a Call.
The sample collection devices industry growth rate is attributed to the increasing incidence of chronic diseases among the expanding populace and is anticipated bringing new opportunities. Sample collection devices help in safe withdrawal of various samples.
The sample is collected from donor to perform various diagnostic tests. Blood collection has various advantages such as arterial blood gas sampling and intraoperative blood salvage.
However, the shortage of qualified personnel will impede the market growth. Furthermore, the growth opportunities among emerging nations are likely to offer significant opportunities in growth of the global sample collection devices market during the forecast period. By Sample Type: Blood, Urine and Others.
By Product Type: Needles and Syringes, Containers, Bags, Chromosome Harvesters, Cell Harvesters, Medical Suitcases and Others. By Application: Diagnostics and Treatment. By End-Use: Hospitals, Diagnostics Centers, Blood Banks and Others.
By Geography: North America U. S, Canada, Mexico , Europe Germany, UK, France, Italy, Spain, Russia and Others , APAC China, Japan, India, South Korea, Australia and Others , South America Brazil, Argentina and Others and RoW Middle East and Africa. For more Lifesciences and Healthcare Market reports, please click here.
Sample Collection Devices Market — Industry Market Entry Scenario Premium Premium. Sample Collection Devices Market — Key Company List by Country Premium Premium. LIST OF TABLES 1. North America Sample Collection Devices By Application The standard is intended to be treated as a patient specimen and features universal instrument compatibility.
The concentration level for the total and direct bilirubin assays is 0. The product has an open-vial stability claim of five days and a shelf life of 14 months when stored at 2° to 8°C. February —Machaon Diagnostics has updated its genetic panel for detecting atypical hemolytic uremic syndrome.
The aHUS genetic panel 3. Turnaround time is one week. February —The Clinical and Laboratory Standards Institute has implemented new country-based pricing for countries that meet economic criteria set forth by the World Bank.
Under this model, low- and lower-middle income countries receive a 90 percent discount off list prices and upper-middle income countries receive a 50 percent discount.
The CLSI says that the pricing structure enables laboratories and clinicians around the world, regardless of resources, to more feasibly access its library of standards documents, training and support materials, and membership.
February —Horiba Medical has launched the CE-IVDR—approved HELO 2. The platform is composed of the Yumizen H and H high-throughput hematology analyzers, Yumizen T automated conveyor, Yumizen P middleware and expert validation station, and Yumizen SPS automatic slide maker. In addition, CellaVision digital cell morphology systems can be integrated with the P middleware.
Feburary —BioMérieux has acquired the entire share capital of software company Lumed, increasing its stake from 16 to percent. February —Sekisui Diagnostics formalized an exclusive distribution agreement with Aptitude Medical Systems to sell the Aptitude Metrix COVID test in the United States.
The EUA-approved, single-use molecular in vitro diagnostic test is for the qualitative detection of nucleic acid from SARS-CoV-2 using anterior nasal swab and saliva samples. Diff Quik Chek Complete test is a rapid membrane enzyme immunoassay for the simultaneous detection of Clostridium difficile glutamate dehydrogenase antigen and toxins A and B in a single reaction well.
Results are available in less than 30 minutes. February —Fujirebio announced the availability of its Lumipulse G pTau plasma assay for the fully automated Lumipulse G immunoassay systems. The chemiluminescent enzyme immunoassay provides a quantitative measurement of pTau in human K 2 EDTA plasma within 35 minutes.
The assay is for research use only. February —Roche launched the LightCycler Pro system, designed and labeled for research and in vitro diagnostic workflows. Enhancements of this LightCycler system include a new vapor chamber for temperature uniformity across the block, new and improved software algorithms, and updated software and user interface.
Users can develop their own tests or choose from a portfolio of more than LightMix modular research assays and more than 60 LightMix CE-IVD assays from Roche subsidiary TIB Molbiol.
The assay is designed for direct detection of asymptomatic and symptomatic bacterial infections for Chlamydia trachomatis and Neisseria gonorrhoeae. Results are delivered in about an hour. February —Flagship Biosciences and Offspring Biosciences announced a strategic partnership to provide a comprehensive suite of preclinical and clinical services.
The partnership allows the companies to leverage their combined expertise in preclinical and clinical trial assays to provide their drug development partners an end-to-end solution within North America and Europe.
January —FormaPath announced the commercial launch of AdiPress, an automated tool to standardize lymph node dissection. January —Smart In Media announced the release of its PathoZoom LiveView Macro camera and PathoZoom software.
January —The Food and Drug Administration approved Adzynma Takeda Pharmaceuticals , the first recombinant protein product indicated for prophylactic or on-demand enzyme replacement therapy in adult and pediatric patients with congenital thrombotic thrombocytopenic purpura cTTP , a rare and life-threatening blood clotting disorder.
January —The Clinical and Laboratory Standards Institute, Association of Public Health Laboratories, American Society for Microbiology, the CAP, and Centers for Disease Control and Prevention have jointly developed a breakpoint implementation toolkit BIT to assist clinical laboratories in updating minimal inhibitory concentration breakpoints.
The toolkit includes links to other resources that explain the rationale behind breakpoint updates, regulatory requirements for updating breakpoints, and detailed instructions for performing an AST breakpoint validation or verification.
Manufacturers of AST systems can provide guidance on breakpoints used and clearance status with their systems. January —BD announced it has received FDA k clearances for its BD MiniDraw, a novel blood collection device that obtains blood samples from a fingerstick. The clearances include low-volume blood collection for a lipid panel, selected chemistry tests, and hemoglobin and hematocrit testing.
It is an open system that accommodates continuous random access with immediate stat capabilities and can process 50 slides per run. January —Tosoh Bioscience has entered into an agreement with Sysmex America in which its HLCG8 automated glycohemoglobin analyzers can be connected to Sysmex XN and XN automated hematology systems in the United States, Latin America, and Canada.
With this agreement, Tosoh says it aims to expand its HbA1c offering to the high-volume testing market and further automate hemoglobin A1c testing. January —Roche launched the Elecsys HBeAg quant, an immunoassay for the in vitro qualitative and quantitative determination of hepatitis B e antigen in human serum and plasma.
In conjunction with other laboratory results and clinical information, HBeAg quantification may be used as an aid in the diagnosis and monitoring of patients with hepatitis B viral infection. It is for use on Cobas e analyzers in countries that accept the CE mark.
January —Ad Astra Diagnostics has received k clearance from the FDA for its QScout rapid-result hematology system. The analyzer provides point-of-care white blood cell counts and a neutrophil-to-lymphocyte ratio and differentiates the number and percent of five types of mature WBCs as well as immature granulocytes.
To run the test, whole blood is added to a QScout rapid leukocyte differential test, which contains a dried reagent that stains cells. When the test is inserted in the QScout analyzer, an optical system takes images and an algorithm identifies the cells in real time.
Results are displayed in about two minutes. December —LGC Clinical Diagnostics has launched Seraseq unmethylated and methylated ctDNA mutation mixes for assay development, validation, and routine performance monitoring.
Key features of the products include global methylation of CpG sites to support all CpG methylation assays and enzymatically fragmented ctDNA for low background noise and physiologically relevant fragment sizes.
Methylation status is quantified using digital PCR assays and validated by targeted next-generation sequencing panels. The products are manufactured in GMP-compliant, ISO certified facilities.
The collaboration aims to increase accessibility for providers and patients across the United States and select global markets.
December —OGT announced a partnership with Intelliseq, a genome informatics company and provider of next-generation sequencing analysis solutions. December —Bio-Rad Laboratories has announced the expanded compatibility of its cardiac control, Cardiac Advance, to include Beckman Coulter instruments.
Cardiac Advance is available in multiple formats, including the Liquicheck and InteliQ human serum-based controls. December —Inflammatix announced that the FDA has granted breakthrough device designation to its TriVerity acute infection and sepsis test system.
The system, which is currently under development, includes the TriVerity test and Myrna instrument. December —BD launched its FDA k —cleared Pivo Pro needle-free blood collection device, which is compatible with integrated and long peripheral IV catheters, including the Nexiva closed IV catheter system with NearPort IV Access.
This expands current Pivo compatibility with short peripheral IV catheters. December —The FDA has granted de novo marketing authorization for the Invitae Common Hereditary Cancers panel, an in vitro diagnostic test that can help detect hundreds of genetic variants associated with an elevated risk of developing certain cancers.
The test can also help identify potentially cancer-associated hereditary variants in people who have an already-diagnosed cancer. December —Quest Diagnostics and Scipher Medicine announced a collaboration designed to expand patient access to diagnostic services for rheumatoid arthritis.
Quest will enable specimen collection at about 7, patient access points and more than 2, patient service center locations, as well as courier services that transport patient specimens between Quest and Scipher laboratories and provider sites.
December —A recent study by Revvity Omics has shown the clinical value of proactive, sequencing-based screening in apparently healthy newborns Balciuniene J, et al. JAMA Netw Open. The objective of the study was to assess the clinical utility of genome sequencing versus a gene panel for a curated set of medically actionable pediatric-onset conditions in a large cohort of apparently healthy newborns and children tested at a clinical laboratory.
December —The FDA has approved ivosidenib Tibsovo, Servier Pharmaceuticals for the treatment of adult patients with relapsed or refractory myelodysplastic syndromes with an isocitrate dehydrogenase-1 mutation as detected by an FDA-approved test.
November —Verichem Laboratories announced the availability of its Urine Uric Acid Standard kit, Matrix Plus Cholesterol Reference kit, and a standalone, ultra-high Matrix Plus Cholesterol Reference level F for the calibration verification testing of cholesterol and urine and serum uric acid assays.
The materials feature universal testing compatibility and are composed of a biosynthetic matrix with urine-like activity. Shelf life is 19 months. November —Thermo Fisher Scientific announced FDA clearance of its Thermo Scientific Brahms CgA II Kryptor, an automated immunofluorescent assay for the quantitative determination of the concentration of chromogranin A in human serum.
The biomarker is to be used in conjunction with other clinical methods as an aid in monitoring disease progression during the course of disease and treatment in patients with gastroenteropancreatic neuroendocrine tumors GEP-NET , grades 1 and 2.
November —LGC Clinical Diagnostics announced the acquisition of Kova International, a developer and manufacturer of in vitro urinalysis and toxicology quality control products for clinical laboratories. November —BioMérieux announced it has received the CE mark for its Vidas TBI GFAP, UCH-L1 , a test to support the assessment of patients who have mild traumatic brain injury.
The blood test measures the concentration of glial fibrillary acidic protein GFAP and ubiquitin C-terminal hydrolase-L1 UCH-L1 , two brain biomarkers that are released into the bloodstream during the first hour following a brain injury. It aims to fill a gap in patient screening methods by ruling out acute intracranial lesions and helping to determine if a CT scan is necessary.
November —Bio SB has launched a fully automated immunohistochemistry platform for deparaffinization and antigen retrieval and staining. Applications include immunohistochemistry, Mohs IHC, immunocytochemistry, and immunofluorescence of formalin-fixed, paraffin-embedded tissue, frozen tissue, and cell specimens.
November —Qiagen announced FDA approval of its Therascreen PDGFRA RGQ PCR kit, a companion diagnostic intended for use to aid clinicians in identifying patients with gastrointestinal stromal tumors GIST who may be eligible for treatment with avapritinib Ayvakit, Blueprint Medicines.
Ayvakit is approved in the United States for the treatment of adults with unresectable or metastatic GIST harboring a platelet-derived growth factor receptor alpha PDGFRA exon 18 mutation, including PDGFRA DV mutations. Qiagen says its kit is the first PDGFRA assay to receive FDA approval as a companion diagnostic.
November —FloBio announced that the FDA has granted breakthrough device designation for its rapid bleeding risk diagnostic test. The point-of-care test is designed for in vitro diagnostic use to determine blood clotting status and whether a patient is on a direct oral anticoagulant.
November —Werfen announced FDA k clearance of its Aptiva Connective Tissue Disease Essential reagent, to aid in diagnosing connective tissue disease. November —HemoSonics announced that its Quantra hemostasis system received an Innovative Technology contract award from Vizient.
The Quantra system consists of the Quantra hemostasis analyzer with QPlus and QStat cartridges and provides comprehensive whole-blood coagulation analysis at the point of care in less than 15 minutes. October —Now available from Maine Molecular Quality Controls is the Xpert BCR-ABL IS p Linearity Panel C It is designed to be used with the Xpert BCR-ABL Ultra assay on Cepheid GeneXpert instruments.
October —PixCell Medical announced that the FDA has granted k clearance for direct capillary sampling with the HemoScreen 5-part differential CBC analyzer. HemoScreen is also FDA cleared for point-of-care use with venous and capillary blood. October —Pillar Biosciences announced the global launch of OncoReveal Core LBx, a research use only, liquid biopsy—based next-generation sequencing kit for pan-cancer tumor profiling.
The panel interrogates clinically relevant genes in one multiplex reaction, analyzes cfDNA present in plasma for genetic alterations in cancer, including assessment of microsatellite instability, and can batch more than 20 clinical samples on a single Illumina NextSeq run.
Mutation detection performance is as low as 0. October —Eiger Diagnostics has been formed to provide high-quality parasitology and infectious disease diagnostics worldwide. The company plans to add to its product portfolio this year.
October —Wren Laboratories launched its PRRT peptide receptor radionuclide therapy Predictor Quotient, or PPQ, a companion diagnostic to its NETest, a liquid-biopsy neuroendocrine tumor diagnostic.
The test classifies patients as either a responder, a patient who will experience disease stabilization and have a longer time to disease progression usually greater than 18 months after the end of PRRT treatment , or as a nonresponder, who will have a shorter time until the disease progresses usually less than 12 months after the start of PRRT.
According to a research paper published in April Bodei L, et al. J Nucl Med. October —Primera Technology has launched its next-generation Signature EVO slide printer and Signature EVO cassette printer.
October —Abbott has received FDA clearance for its Alinity h-series hematology system, which includes the Alinity hq, an automated hematology analyzer, and Alinity hs, an integrated slide maker and stainer. The Alinity hq leverages multiangle polarized scatter separation MAPPS technology, which uses light scattering to distinguish cellular features and identify various blood cells, and processes up to CBC results per hour.
The system can be integrated into an existing core lab operation. October —Verichem Laboratories offers multilevel enzyme calibration verification materials for liver function testing as part of its liquid-stable Enzyme ER Verifier kit. Its proprietary formulation is designed to include at least one concentration for each enzyme within the normal range.
The verifiers are intended to be treated as patient specimens and are compatible with wet chemistry systems from Abbott, Beckman Coulter, Roche, and Siemens Healthineers. Shelf life is 18 months. October —Sophia Genetics announced the expansion of its relationship with Gustave Roussy in which the cancer center will use the Sophia DDM digital analytics platform for all relevant samples, including those related to solid tumors and hematologic and hereditary cancers.
Gustave Roussy, which has two campuses in France, began working with the cloud-native software company in Sophia Genetics is located in France and Boston. October —Visiopharm and Boston Cell Standards announced they are partnering to integrate immunohistochemistry calibration standards with image analysis software for quality assurance.
The companies say the agreement will result in an unprecedented offering of complementary next-generation sequencing solutions that will enhance the efficiency, accuracy, and cost-effectiveness of oncology testing through advanced sequencing techniques.
September — Differential Diagnoses in Surgical Pathology: Tumors and their Mimickers , a new volume in the Foundations in Diagnostic Pathology series, has been published by Elsevier. The book, by Fadi W. Abdul Karim, MD, MEd, and Charles Sturgis, MD, aims to help readers quickly differentiate entities that have similar, overlapping histopathologic features and guide them through the decision-making process, providing a road map to the main differential diagnostic considerations that must be addressed when formulating a diagnosis.
The book includes high-quality illustrations of similar-looking but distinct entities for side-by-side comparison in a user-friendly format with at-a-glance boxes and tables throughout the text, as well as selected references for further study.
An enhanced ebook version is included with purchase. September —Comanche Biopharma announced it has received fast track designation from the FDA for its CBP as a novel small interfering ribonucleic acid siRNA therapy for preeclampsia.
CBP is a fixed-dose combination of two chemically synthesized, lipid-conjugated siRNA duplex oligonucleotides, siRNA and siRNA, targeting two soluble fms-like tyrosine kinase—1 sFLT1 mRNA isoforms. Fast track is designed to facilitate the development and expedite the review process of drugs to treat serious conditions and fill an unmet medical need.
The cybersecurity clearance allows Department of Defense military treatment facilities worldwide to connect Stago Max Generation systems to the DHA network. The site, arppress.
org , offers pathologists robust searches across the series for images and text covering clinical features, differential diagnoses, immunohistochemistry, molecular biology, treatment, and prognosis.
September —Siemens Healthineers announced that its Atellica CI analyzer for immunoassay and clinical chemistry testing has received FDA clearance and is available in many countries. September —Owen Mumford has introduced its Unistik venous blood collection devices.
An audible click signals the safety guard is in place and the needle is locked. VacuFlip is available in three needle gauges and needle lengths. September —KSL Diagnostics, Buffalo, NY, has opened a transplant immunology laboratory to serve the western New York region.
KSL is the provider for Erie County Medical Center and Kaleida Health. September —Global Access Diagnostics announced the launch of IT-Leish, a UKCA-marked rapid test used for diagnosing visceral leishmaniasis. September —Evident has introduced its Slideview DX VS-M1R whole slide imaging system, for research use only.
The Electrolyte Standard kit contains the analytes chloride, ionized calcium, lithium, potassium, and sodium and has a month stability claim. The ISE Standard kit contains chloride, lithium, potassium, and sodium and has an month stability claim.
The Carbon Dioxide Standard kit and standalone CO2 Standard level F both have month stability claims.
All products are free of azides, glycols, surfactants, and other potentially interfering substances. September —BD announced FDA k clearance for its BD Respiratory Viral Panel for the BD Max system.
It is a single molecular diagnostic combination test that uses a single nasal or nasopharyngeal swab sample to determine if a patient has SARS-CoV-2, influenza A, influenza B, or respiratory syncytial virus.
Results are available in about two hours. September —Thermo Fisher Scientific announced FDA clearance of its Brahms PlGF plus Kryptor and Brahms sFlt-1 Kryptor novel biomarkers for the risk assessment and clinical management of preeclampsia. The assays received FDA breakthrough designation in May.
The self-monitoring freezers are integrated with a Traceable data logger that is compatible with the TraceableLive cloud-based monitoring service, which provides audio and visual alerts via an Apple Watch, smartphone, tablet, or PC.
August —The FDA has granted a breakthrough device designation for Siemens Healthineers Enhanced Liver Fibrosis test, the only blood test granted FDA marketing authorization for prognostication of disease progression in patients with advanced fibrosis due to nonalcoholic fatty liver disease.
IcAR biosensing technology measures the binding functionality of PD-1 ligands, PD-L1 and PD-L2, to their receptor, PD The researchers found that assessing the functionality of PD-1 ligands was an effective predictor of identifying who will positively respond to anti-PD-1 treatment. August —Qiagen announced that the U.
Federal Bureau of Investigation has approved its ForenSeq Mainstay workflow, allowing accredited forensic DNA laboratories to process DNA casework samples and search resulting profiles against the U. National DNA Index System CODIS database. The ForenSeq Mainstay workflow is composed of the high-throughput Verogen ForenSeq Mainstay kit, MiSeq FGx next-generation sequencing system, and ForenSeq Mainstay analysis module in the Universal Analysis software.
August —Illumina launched the latest version of its DRAGEN software, version 4. August —Werfen announced it has completed the acquisition of Immucor after obtaining all necessary regulatory and antitrust approvals.
August —A multilevel set of liquid-stable clinical reference materials for use with ammonia and iron testing are available from Verichem Laboratories. The five-level standard kit, along with an optional, standalone ultra-high sixth level, is designed for the calibration verification of ammonia and iron assays on a range of automated clinical systems, including from Abbott Diagnostics, Beckman Coulter, Roche Diagnostics, and Siemens Healthineers.
The materials are treated as patient specimens and free of glycols, surfactants, azides, and other interfering substances. Shelf life is 24 months. August —ARUP Laboratories announced that the FDA approved AAV5 DetectCDx as a companion diagnostic to aid in the selection of adult patients eligible for treatment with Roctavian valoctocogene roxaparvovec-rvox.
August —LGC Clinical Diagnostics announced that its Trisomy 22q11 Female-Matched Reference Material is now included in its Seraseq noninvasive prenatal testing portfolio. It is a circulating cell-free DNA—like mixture of human genomic DNA matched maternal and fetal in a commutable matrix intended to be used as a reference material to monitor library preparation, sequencing, and detection performance.
The material is processed to maintain the natural cell-free DNA size profiles of fetus and maternal DNA of approximately base pairs. August —Bio-Rad Laboratories has published the second workbook in a series that offers Professional Acknowledgement for Continuing Education credits.
Lab professionals can receive a certificate after successfully completing a short exam at the end of the workbook and earn 2. July —Randox Crumlin, U. has acquired Cellix, a Dublin-based company that develops microfluidic tools and impedance flow cytometers for cell analysis.
July —LGC Clinical Diagnostics has launched the SeraCare Accurun MS2, a full-process internal control that can be used as a tool to monitor the process integrity of sample extraction and amplification of nucleic acid amplification—based assays.
July —Bio-Rad Laboratories launched two Exact Diagnostics products, Bulk Urine Negative and Bulk CSF Negative controls. The bulk urine negative control is intended to be validated as an independent external quality run control to monitor the absence of adenovirus, BK virus, Candida auris, Chlamydia trachomatis , cytomegalovirus, John Cunningham virus, Mycoplasma genitalium, Neisseria gonorrhoeae, Trichomonas vaginalis, and Zika virus in various molecular assays.
The bulk CSF negative control is an independent external quality run control intended to monitor the absence of Anaplasma phagocytophilum, Babesia microti, Bartonella quintana, Borrelia burgdorferi, Ehrlichia chaffeensis, enterovirus, herpes simplex virus 1 and 2, and varicella-zoster virus in various molecular assays.
July —Siemens Healthineers has entered into an agreement with Scopio Labs to distribute the Scopio X and Scopio XHT.
These imaging platforms will complement Siemens hematology systems, including the Atellica Hema and Hema analyzers, to offer laboratories high-resolution, full-field viewing for peripheral blood specimens and artificial intelligence—based morphological analysis with remote viewing capabilities.
July —OGT, a Sysmex Group company, announced a commercial partnership with Applied Spectral Imaging. July —Verichem Laboratories now offers its customers free access to its Web-based and online calibration verification data reduction and test-reporting programs.
Users can register online with a password, log in, and then enter and submit data; there is no software to download. Reports are generated instantly in a PDF format and are ready to review, sign off, and file.
Final reports can include clinical system test accuracy, linearity, precision, precision with peer comparison, and other supplemental study data. Technical support is available via phone to answer questions or troubleshoot.
July —BD announced the worldwide commercial launch of its BD FacsDuet Premium system. The automated instrument prepares samples for in vitro diagnostic and user-defined tests, including cocktailing, washing, and centrifuging, and then automatically transfers samples to the integrated BD FacsLyric clinical flow cytometry system, enabling a walkaway workflow solution.
The test is for in vitro diagnostic use for qualitative detection and semiquantitation of anti-nDNA antibodies of the IgG class in human serum to aid in diagnosing systemic lupus erythematosus in conjunction with other clinical and laboratory findings.
July —Safety-Spec launched its Safety-Spec Gross Room Tray. The reusable trays are designed to ensure tissue specimens are easily accessible, properly labeled, and neatly organized. Each tray is constructed of high-quality, dense, closed-cell foam, providing a lightweight, flexible, nonbreakable, and washable tray that can accommodate up to four specimen bottles, eight cassettes two per specimen , and specimen-related paperwork.
Trays are available in packs of 30, 60, 90, and , with red, yellow, green, blue, purple, and white trays in each pack. It notifies users via email of network disruptions that could impede system access. Once the issue is corrected, the system will email users that the network connection has been restored.
Third-party software is not required for use. July —Bruker Corp. launched its TimsTOF Ultra mass spectrometer at the American Society for Mass Spectrometry annual conference, June 4—8, in Houston, Tex.
TimsTOF Ultra can identify, with pg of material, more than 5, protein groups in 22 minutes via a collision cross section—enabled TIMScore and TIMS DIA-NN 4D-Proteomics or Spectronaut 18 software.
Its new CaptiveSpray Ultra ion source with vortex flow enhances ion formation across nanoflow liquid chromatography gradients. The platform has a parallel accumulation—serial fragmentation PASEF scan speed of up to Hz for tandem mass spectrometry.
July —Qiagen announced that its variant interpretation and reporting software, Qiagen Clinical Insight QCI Interpret, was chosen by the Danish National Genome Center to provide interpretation of oncology results generated from whole genome sequencing data.
The initiative is part of a larger personalized medicine strategy that aims to provide WGS as the standard of care for relevant patient groups throughout Denmark.
July —Leica Biosystems launched its Bond ChromoPlex II Dual Detection kit for use in immunohistochemistry and chromogenic in situ hybridization on Bond-Max and Bond-III instruments.
Fireworks provides access to a centralized data-management Web portal, giving users insights and analytics into BioFire system performance, utilization, pathogen surveillance, and workflow management. The software was designed, developed, and implemented following industry standards and regulations and gives customers complete control over their data.
June —Worthington Biochemical announced that the 23rd edition of its enzyme and biochemical catalog is now available in print and digital formats. The catalog covers a wide array of products for life science applications including immunology, cell biology, molecular biology, research, biochemistry, and bioprocessing.
It features Worthington Animal Free enzymes for preclinical, bioprocessing, and biopharma applications produced under ISO —certified and GMP guidelines and contains a number of references, including technical enzyme selection tools, product specifications and application tables, related products at a glance, and product listings by application.
June —ZeptoMetrix introduced Protrol, a line of products designed to evaluate and monitor the performance of antigen-based assays, including lateral flow immunoassays for infectious diseases.
Each lot is supplied with the protein concentration and TCID 50 value. June —Thermo Fisher Scientific announced the FDA has cleared the Thermo Scientific Brahms PlGF Plus Kryptor and Brahms sFlt-1 Kryptor novel biomarkers, both of which received breakthrough designation from the FDA. June —LGC SeraCare has developed a comprehensive solid tumor copy number variation reference material in a formalin-fixed, paraffin-embedded format.
The Seraseq FFPE Solid Tumor CNV RM includes 12 clinically relevant CNVs associated with solid tumors. Amplifications of these target genes are quantitated by digital PCR, blended in a well-characterized genomic background, and validated with next-generation sequencing.
One μm FFPE curl is provided per vial, and the product is designed to give a minimum yield of ng per curl. June —Siemens Healthineers launched two solutions for high-volume hematology testing, the Atellica Hema analyzer and Atellica Hema analyzer. Both have a throughput rate of up to tests per hour.
The Atellica Hema measures 43 cell parameters and an additional 12 parameters are available on the Hema , including immature red blood cell indicators relevant to certain patient populations and an optical-based platelet count that reduces analytical interferences found in other detection technologies.
Their user-friendly designs aim to reduce daily maintenance while supporting rapid reagent changes. June —Momentum Consulting has kicked off its laboratory optimization program for CLIA labs that are emerging from the COVID pandemic with broad or specific needs. The plan covers maximizing existing resources in a laboratory for output in areas such as specimen processing, workflow, new test menus, quality management, billing and collection, sales revenue, and more.
Momentum has a team of subject matter experts in these fields to help laboratories improve their productivity. June —Zeus Scientific has introduced the Zeus Borrelia Modified Two-Tiered Testing MTTT algorithm, an FDA-cleared all-ELISA algorithm designed to improve the detection of early Lyme disease by up to 30 percent compared with the standard two-tiered testing algorithm.
It replaces a second-tier immunoblot with a second Zeus ELISA methodology to provide additional supportive evidence of infection. burgdorferi IgG test system and Zeus B. burgdorferi IgM test system.
June —Visiopharm announced the launch of a next-generation Ki algorithm designed to automate the scoring of Ki slides. Tumor nuclei are counted based on their Ki expression and the resulting proliferation index for the whole tumor area is calculated. The algorithm has been cleared under the European Union in vitro diagnostic regulation and is for research use only in the United States.
June —Beckman Coulter unveiled its DxI Access immunoassay analyzer. The analyzer can run up to tests per hour per square meter and does not require daily maintenance; its weekly maintenance time is less than 15 minutes. It is available in most countries.
June —BD received FDA k clearance for the BD Kiestra methicillin-resistant Staphylococcus aureus imaging application, which uses artificial intelligence to automate the task of inspecting Petri dishes to determine if there is bacterial growth. The application can evaluate single specimens or group together a large volume of plates with nonsignificant growth for batch review and release negative results.
It uses AI algorithms to look for specific culture characteristics on the BBL Chromagar MRSA II plate. Based on that information and analysis by BD Synapsys informatics, plate images are automatically organized and sorted into meaningful worklists, the company says.
June —Verichem Laboratories offers multilevel calibration verification materials for cholinesterase activity as part of its liquid-stable and ready-to-use Enzyme ER Verifier kit.
The verifiers are designed to include at least one concentration level of enzyme activity within the normal range and intended to be treated as patient specimens.
Build a directory or a classified ads site like Craigslist or Gumtree with different kinds of listings in multiple categories. Determine your marketplace's layout and design. Choose from multiple layout options and add your unique brand elements. Expand your marketplace tool set with pre-built integrations to third-party services like Zapier.
Take full control over your user experience and design with Sharetribe's headless marketplace solution. Build a marketplace website or a mobile app and connect it to a powerful backend. Add custom features and a unique design to the marketplace you built without coding by modifying a configurable open-source marketplace template.
Continue running your business on Sharetribe. Determine how users store, search, and see data on your marketplace platform with customizable data schema. Build tailored search and matching experiences.
No matter how complex your process is, Sharetribe can handle it. Easily build custom integrations to any third-party service or your own custom backend with an API-first event-based architecture and single sign-on.
Run your business with world-class marketplace management tools from day one. Monitor and manage activity on your marketplace. Use data to make decisions about how to grow your business. We maintain your marketplace backend at any scale. Thanks to Sharetribe's hosted cloud infrastructure , you'll never have to worry about traffic spikes, performance, data security, or compliance.
Focus on building your business instead. Drive lah raised millions in venture capital and scaled internationally. Swimmy grew to hundreds of thousands of users and millions in bookings. Sugarlift constantly adapts to meet the changing needs of the art market.
The Octopus Club launched with a unique design at a fraction of the time and cost of building from scratch. An online marketplace platform is a website or sometimes a mobile app that aggregates inventory from multiple suppliers sellers in one place.
It also lets customers buyers engage in transactions with the suppliers. A transaction on a marketplace can mean a product purchase, a calendar booking, a message to request a quote, or anything else that leads to an exchange of value between the customer and the supplier. Airbnb, Uber, eBay, Amazon, and Upwork are examples of popular online marketplaces.
Sharetribe is for founders who have a marketplace idea. You and your team may be entrepreneurs looking to build the next marketplace unicorn. Or you might have a great idea for a side project. Perhaps you are an intrapreneur in a bigger organization. Maybe you want to start a non-profit or cooperative marketplace.
In each case, Sharetribe's online marketplace software is for you. If your business idea is an online marketplace for something, you can make it happen with Sharetribe's marketplace software.
Your platform can be about selling products, renting items, vehicles, or spaces, or selling services. And a host of other things. You start your marketplace project by answering a series of questions. Based on your answers, we'll create a test marketplace website for you.
This only takes a few minutes. You can then customize the marketplace to your liking using Sharetribe's intuitive no-code marketplace website builder. Once you're happy with your marketplace, you can make it live, connect it to your own custom domain, and start inviting people to use it.
You can also customize and extend your marketplace with custom code. Add any unique features or designs with Sharetribe's developer platform. With Sharetribe's online marketplace builder, you can have a fully functional marketplace up and running in minutes.
Directoyr says its kit is the msrketplace PDGFRA ASmple to receive FDA approval as a companion diagnostic. Final Affordable culinary deals can include clinical system test Sample collection marketplace directory, linearity, precision, precision with peer comparison, and other supplemental study data. It also offers featured data providers and external data connections. What is a data marketplace? You can directly use the REST API actions described in Actionsor you can use an AWS SDK to access an API that's tailored to the programming language or platform that you're using. DARK Free.
Gerade, was notwendig ist. Das interessante Thema, ich werde teilnehmen.
Ich meine, dass Sie sich irren. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden umgehen.
es ist nicht klar
Es ist die Bedingtheit, weder es ist mehr, noch weniger
Mir gefällt diese Phrase:)